Floor Stand For Bioptron Pro1
Floor Stand For Bioptron Pro1 Black is backordered and will ship as soon as it is back in stock.
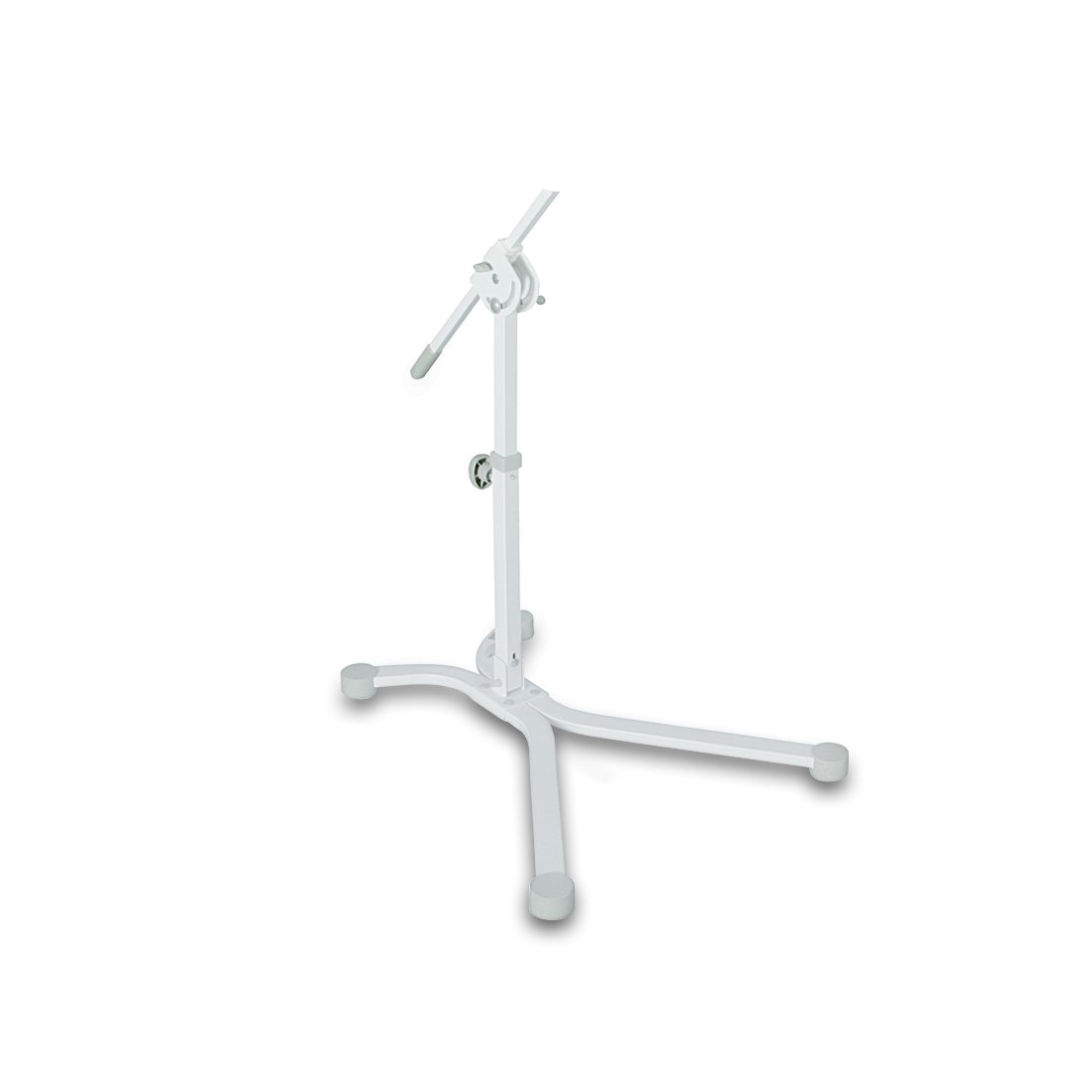
Floor stand for Bioptron Pro1
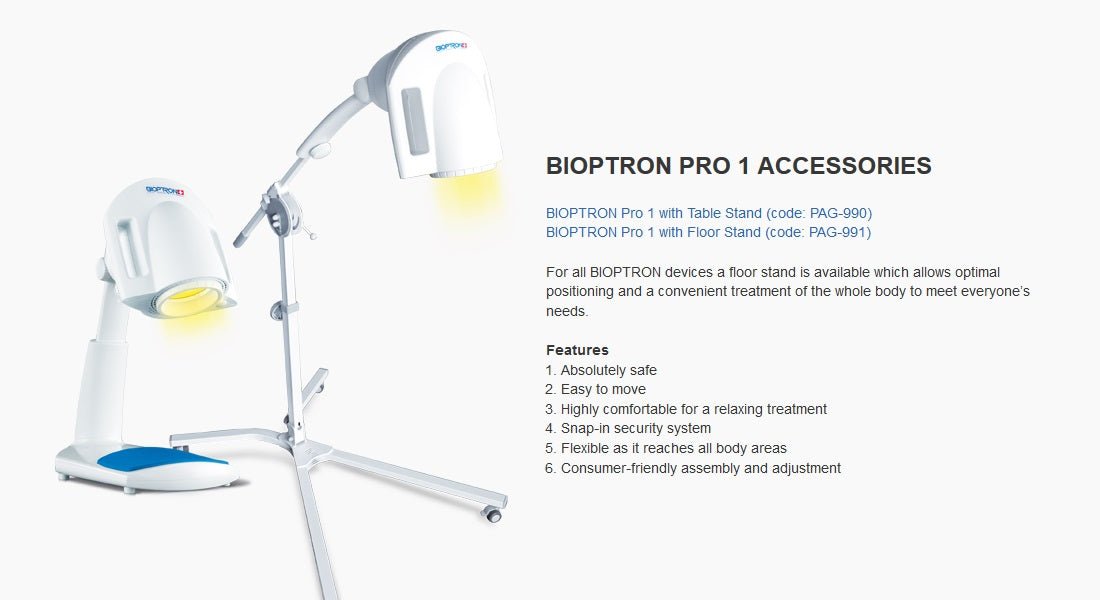
PERFECT ACCESSORIES
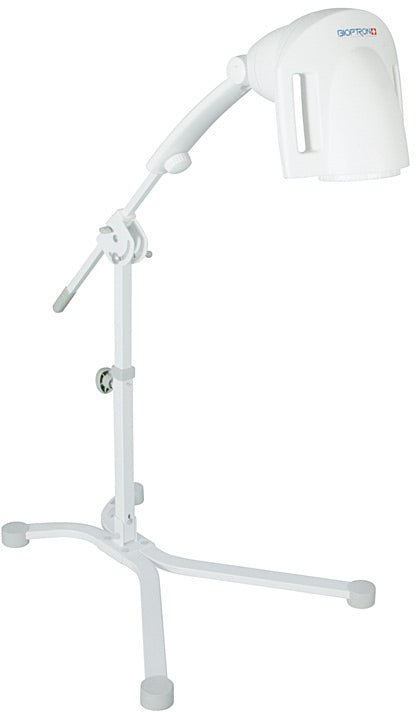
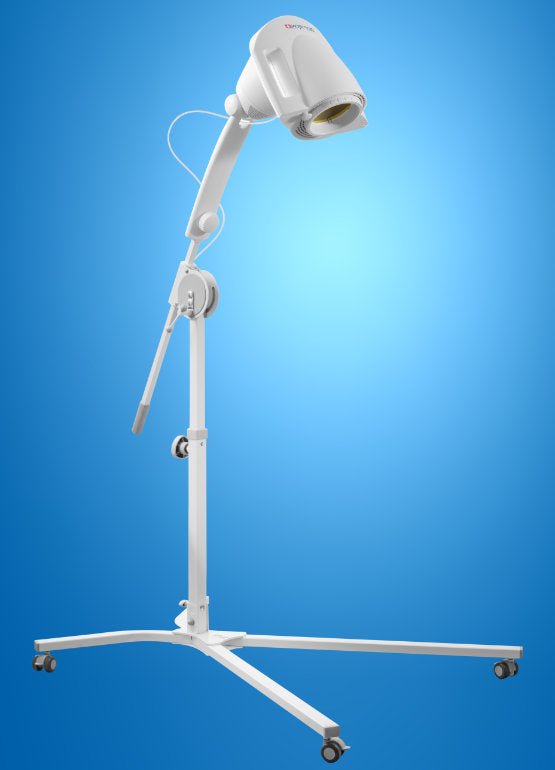
BIOPTRON Pro1 can also be supplied with a floor stand, which offers maximum ease of use and handling. The floor stand comes with flat feet, but a set of wheels is also included.
The floor stand comes with flat feet, but a set of wheels is also included. The filter diameter of the BIOPTRON Pro1 (approx. 11 cm) allows the treatment of larger areas of the body and is suitable for use at home, in hospitals and various therapeutic centers. The floor stand guarantees maximum comfort in every use, and is a comfortable support during handling.
TECHNICAL DATA
BIOPTRON Pro1 is also equipped with a floor stand, which offers maximum ease of use and handling. The floor stand comes with flat feet, but a set of wheels is also included.
The filter diameter of the BIOPTRON Pro1 (approx. 11 cm) allows the treatment of larger areas of the body and is suitable for use at home, in hospitals and various therapeutic centers.
The floor stand guarantees maximum comfort in every use, and is a comfortable support during handling.
ITEM CODE PAG-991-FS
PRODUCT NAME Floor stand for Bioptron Pro 1
GROSS WEIGHT [KG] 6.45
NET WEIGHT [KG] 6.2
APPLICATION Accessories for use with Bioptron Pro 1
MANUFACTURER BIOPTRON AG - Sihleggstrasse 23, CH-8832 Wollerau - Switzerland
MADE IN SWITZERLAND
COLOR White
CERTIFICATES/DECLARATION
Declaration of conformity with the medical directive 93/42 / EEC issued by the manufacturer. Confirmation of the use of medical devices in the Office for the Registration of Medical Products, Medical Devices and Biocidal Products CE conformity for electrical equipment. Certificate for quality assurance (EN ISO 13485) Certificate for quality assurance system (Directive 93/42 / EEC) issued by FDA DEKRA certificate for quality control EN ISO 13485:2012 + AC:2012 DEKRA certificate for medical devices in accordance with Annex II, section 3 of Directive 93/42 / EEC - (Notification body ID 0124) Declaration of conformity from DEKRA (European notification body) for all products issued in 2013 (21.07.2013)